New energy-efficient method for recycling lithium-ion batteries
27.03.2023Researchers at Linnaeus University have developed a more environmentally friendly way of retrieving cobalt from used lithium-ion batteries. With a liquid solvent made of readily available substances, derived from urine and acetic acid, over 97 % of the cobalt can be recovered. The researchers see good potential for large-scale application.
 Researcher Subramanian Suriyanarayanan (left) and Professor Ian Nicholls
Researcher Subramanian Suriyanarayanan (left) and Professor Ian Nicholls
© Joakim Palmqvist, Linnaeus University
A new method for recycling cobalt developed by Ian Nicholls’ research group moves us towards a greener battery industry. The method addresses two major problems with current recycling: high energy costs and dangerous waste.
 New energy-efficient method for recycling lithium-ion batteries
New energy-efficient method for recycling lithium-ion batteries
© Joakim Palmqvist, Linnaeus University
The method involves dissolving the lithium cobalt oxide, a substance used in modern lithium-ion batteries, using a liquid solvent, that separates the cobalt, which can subsequently be used for fabricating new batteries. “The solvent is a combination of two readily available substances: a simple derivate of urea, which is naturally occurring in urine, and acetamide, which can easily be retrieved from acetic acid”, explains Subramanian Suriyanarayanan, one of the researchers behind the solvent that Linnaeus University has researched since 2013. The main benefit of the new solvent, compared to widely used methods for recycling cobalt, is that the process can take place at much lower temperatures.
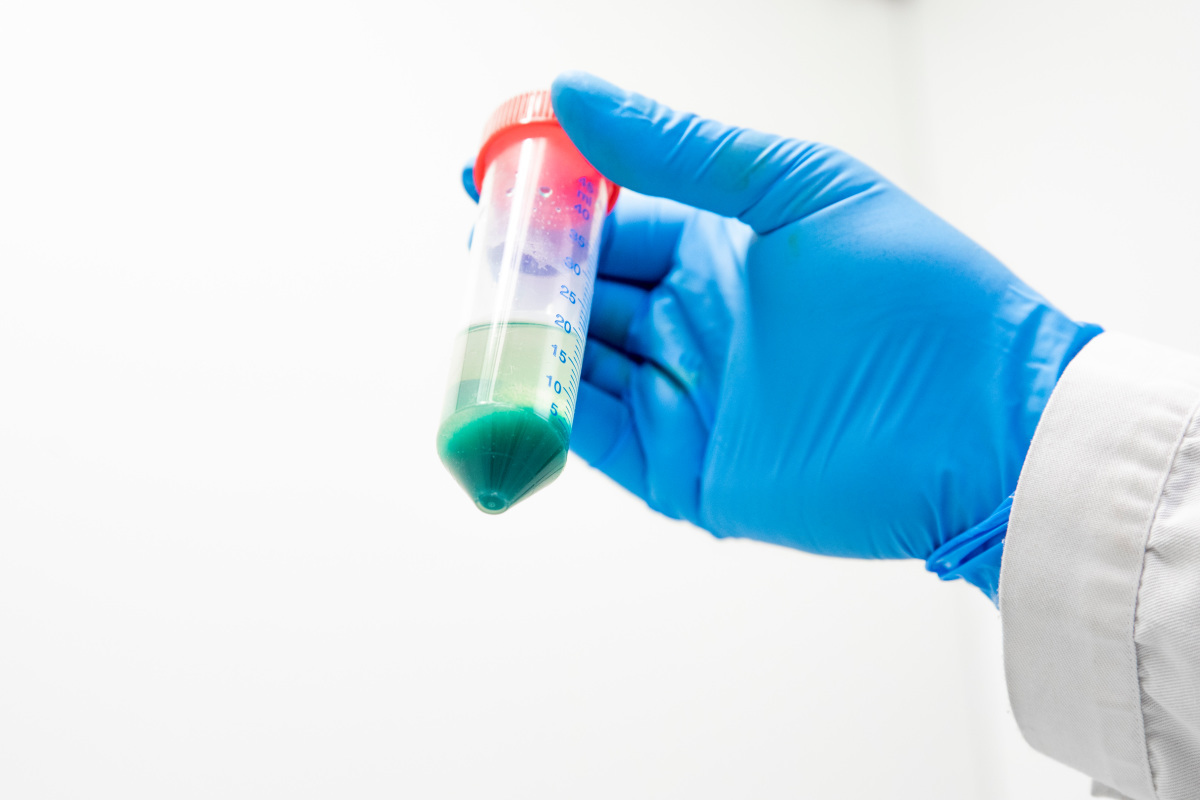 This is what the cobalt looks like once it has been extracted from lithium-ion batteries. Joakim Palmqvist
This is what the cobalt looks like once it has been extracted from lithium-ion batteries. Joakim Palmqvist
© Joakim Palmqvist, Linnaeus University
The researchers have extracted over 97 % of the cobalt from pieces of lithium cobalt oxide that has spent two days in the heated solvent. “In our case, the reaction is as most efficient at 180 degrees Celsius. That makes our method much more energy efficient than today’s commercial options, such as pyrometallurgy, which require extreme temperatures, often exceeding 1400 degrees,” says Ian Nicholls.




